My journey in medicine began as a humble surgical laser technician. This role opened a captivating world that would fuel my curiosity throughout my career.
My first surgtech job took me on travels across the United States, where my position evolved into surgical educator, training ophthalmologists in innovative refractive techniques and laser surgery. It was an incredible opportunity to explore new corners of the country and gain access to advanced technologies and diverse perspectives. In an era before ChatGPT, I sought advice from a multitude of experts and drew my own conclusions, all through analog conversations.
As a young technician and having a strong passion for medical science, I often questioned my involvement in the redundancy of surgical operations. On challenging days when there was 10+ patients all in queue, I questioned my limitations and ability to endure a revolving door of surgery.
But one day in the operating room, something happened that made me realize how important each member of the team is. A careless surgeon, unprepared and driven by temariousness, incorrectly placed a microsection device onto a patient’s eye, inadvertently penetrating the sclera. As the person in the operating room responsible for the technology, I became the guardian of the patient’s well-being. I stopped the surgery before further damage could occur.
That pivotal moment in my career occurred a long time ago, but I will not forget it. Today, as I work in pathology, I am amazed by how the complexities and possibilities have unfolded.
In my previous article, I explored the prevalence of prostate cancer in the United States, where an estimated 1 in 8 men may develop this disease in their lifetime. I emphasized the importance of personalized oncology, early detection, and treatment for improving outcomes.
After submitting that article, I again grappled with limitations and questioned relevance. Around then, the algorithm on my phone began suggesting articles that challenged the concept of personalized medicine, suggesting it might even exacerbate healthcare disparities.
One article stated: “The central focus on the individual and on high-cost technologies that benefit a small portion of the population not only will fail to reduce the main health problems affecting the world, but may also increase the inequalities, with concentration of resources and technologies in the population strata that already have the best access to health, thereby exacerbating health inequalities and hampering health services’ sustainability, especially in low and middle-income countries.”
Was personalized medicine just a sham? This revelation struck a blow to my personal mission of equalizing global cancer diagnostics. It prompted me to embark on an extensive journey involving interviews with molecular pathologists near the digital lab.
Together, we explored several case studies illustrating how a personalized approach to prostate cancer treatment can greatly enhance a patient’s quality of life and potential outcomes.
Two emerging technologies, molecular testing, and digital pathology, hold the potential to revolutionize prostate cancer diagnosis and management. Molecular testing can identify genetic mutations and biomarkers that offer insights into the risk of developing prostate cancer, disease aggressiveness, and the probability of responding to specific treatments. Molecular tests for prostate cancer involve the measurement of gene expression, providing insights into the likelihood of cancer aggression. Various genes associated with cell growth, division, and repair are analyzed. Gene mutations can lead to uncontrolled growth and spread in cancerous cells.
Common genes tested for prostate cancer include:
· ERBB2: This gene influences cell growth and division, and its amplifications are linked to more aggressive prostate cancer.
· PTEN: This gene plays a role in cell growth and repair, and PTEN mutations are linked to more aggressive prostate cancer.
· SPINK1: This gene contributes to semen production, and SPINK1 mutations are associated with less aggressive prostate cancer.
The results of genetic tests are typically reported as a gene expression score or gene mutation status, aiding pathologists in assessing cancer aggression. While not infallible, these tests offer valuable information to guide treatment decisions.
As for Digital pathology, this technology entails the use of high-resolution scanners to generate digital images of pathology tissue slides, subsequently analyzed using computer algorithms and/or digitally sent to an expert GU subspecialist with more experience in prostate biopsy analysis. This innovation enhances the precision and efficiency of pathological diagnoses and enables the development of novel diagnostic tools and algorithms. Most importantly it allows for easy peer review and confirmation by a subspecialist for a more confident diagnosis.
Case Report
A 60-year-old man with a family history of prostate cancer visited his physician for elevated prostate-specific antigen (PSA) levels. The physician referred him to a urologist, who recommended a prostate biopsy. However, before the biopsy, the patient underwent the GoPath ProstateNow test, a molecular analysis that measures the expression of 17 genes in prostate tissue. The results indicated that the patient had a low risk of developing aggressive prostate cancer. Based on these results, the urologist chose to monitor the patient’s PSA levels and perform the biopsy in a year if there was a significant increase.
Seven years later, the patient’s PSA levels finally showed a slight rise, leading to a biopsy. The tissue samples were subjected to digital pathology analysis, which revealed
a small area of prostate cancer confined to the prostate gland. The cancer was slow-growing and had a low Gleason score, suggesting it was unlikely to be aggressive. The urologist recommended continued PSA monitoring and periodic biopsies, with the option of surgery or radiation therapy. However, the patient opted for active surveillance.
Discussion
This case report underscores the potential benefits of utilizing molecular testing and digital pathology in prostate cancer diagnosis and management. The ProstateNow test accurately identified the patient as having a minimal risk of aggressive prostate cancer, allowing the urologist to minimize unnecessary biopsies. Almost a decade later, digital pathology and analysis revealed the presence of slow-growing, localized prostate cancer, information that traditional analog pathology methods may have struggled to provide.
The patient in this case opted for active surveillance, the data from molecular testing and digital pathology analysis helped guide alternative treatment decisions.
Conclusion
Molecular testing and digital pathology are two emerging technologies that hold significant promise in improving prostate cancer diagnosis and management. By delivering more precise information about risk, aggressiveness, and prognosis, these technologies empower clinicians to develop personalized treatment plans for each patient.
Future Directions
As we move forward and diagnostic medicine evolves, molecular testing and digital pathology are expected to play an even more substantial role in the diagnosis and management of cancer. Ongoing research is focused on developing new molecular tests and digital pathology algorithms that can detect even more subtle changes in patient tissue. Combined, these innovations could enhance early detection accuracy, predict cancer progression risk, and identify patients most likely to respond to specific treatments. Overall, molecular testing and digital pathology represent promising technologies with the potential to revolutionize prostate (and other) cancer diagnosis and management.
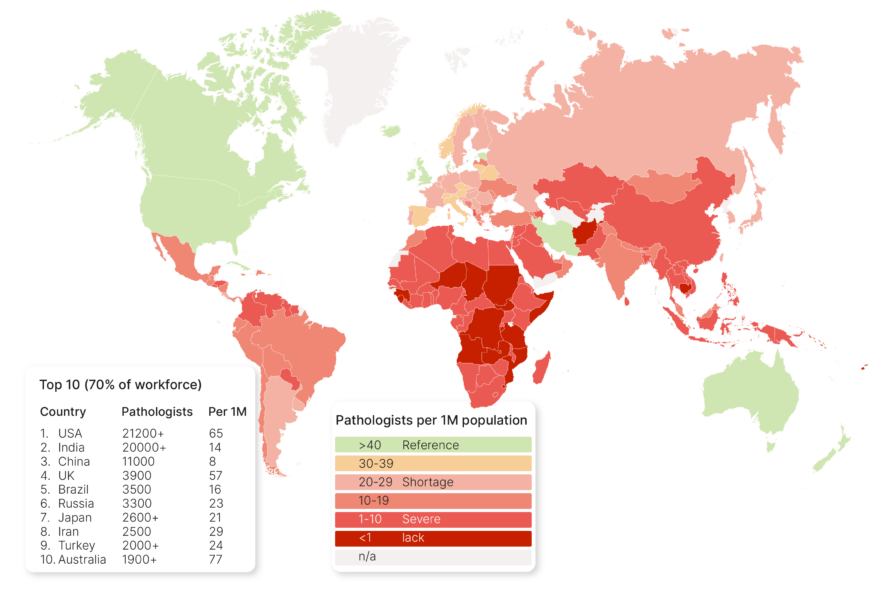
With the notion of personalized oncology no longer viewed as a sham, a new question arises: Can personalized oncology aid patients in low- and middle-income countries (LMIC)? In my next article, I will explore this question in detail. In the meantime, I invite all to share their ideas on how personalized oncology can help equalize cancer diagnostics in LMICs. I have my own theories and am actively working to validate them, but I am eager to hear your thoughts on this pressing matter.