The transformative potential of digital pathology hinges on its ability to enhance diagnostic accuracy and streamline workflows. However, this progress rests on a fundamental pillar: ensuring data integrity and safeguarding patient safety. While our previous article explored quality control and data integrity within largely automated ecosystems, this article delves into the crucial role of quality control (QC) for laboratories embarking on their digital journey.
For newcomers transitioning to digital, a common setup involves connecting a scanner directly to a cloud-based image management system (IMS). While the allure of immediate cloud upload is understandable, bypassing QC altogether is a colossal misstep, akin to navigating uncharted waters without a compass. This oversight is particularly egregious for nascent digital labs with smaller volumes, often characterized by a lack of established protocols and adherence to best practices. Such a blunder is not merely an operational inconvenience; it exposes the laboratory to rampant regulatory scrutiny and risks tarnishing its professional reputation.
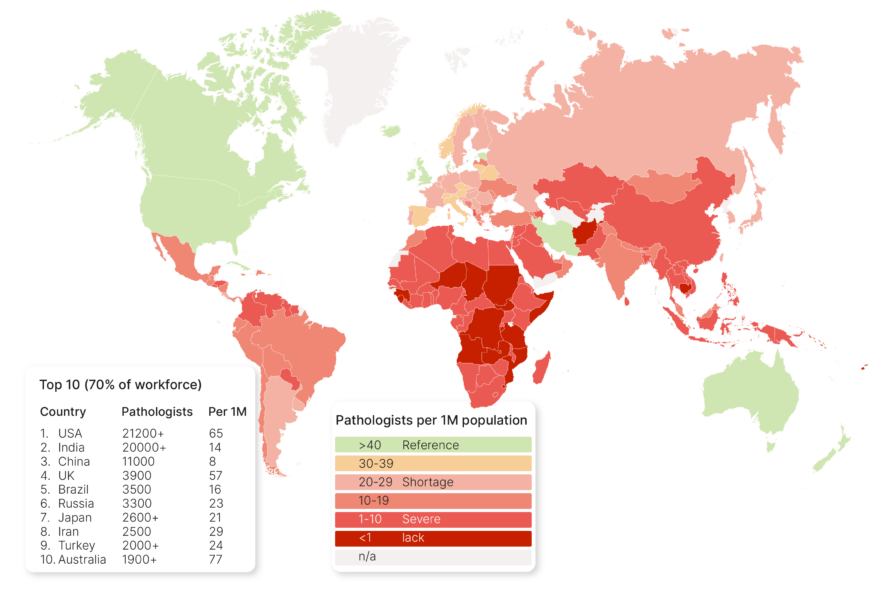
Prevalence of Quality Errors
Wright et al. (2021) investigated the impact of quality control on digital pathology image analysis accuracy. Analyzing 2211 colorectal cancer cases with pathologist-labeled tumor areas, they identified 12 recurring image quality issues (e.g., weak
staining, tissue folds) affecting algorithm performance. Training on the QC-accepted subset, which excludes problematic cases, represented 33% of the original data, indicating that roughly two-thirds of the cases may have contained quality issues to some degree. This study emphasizes the need for robust QC methods as well as improved algorithms used in QC automation. (Wright, Andrew, et al. “The effect of quality control on accuracy of digital pathology image analysis.” IEEE Journal of Biomedical and Health Informatics 25.2 (2021): 307-314.)
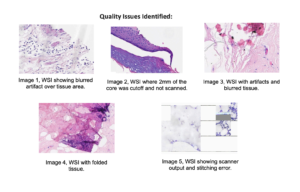
Navigating the Regulatory Minefield:
Emerging regulations, notably in the EU and US, mandate stringent QC for digital pathology data. These regulations, such as 21 CFR part 11 in the US, emphasize data integrity, audit trails, and personnel training. Omission of a documented QC process, whether manual or automated, raises red flags for regulatory bodies such as the FDA, potentially leading to:
• Fines and penalties: Non-compliance with QC regulations can incur hefty fines, impacting the financial health of the laboratory.
• Revoked licenses or sanctions: In extreme cases, blatant disregard for regulatory requirements can lead to suspension or even revocation of operating licenses, posing an existential threat to the laboratory.
• Reputational damage: News of regulatory non-compliance can quickly spread, casting a shadow over the laboratory’s professional image and potentially impacting patient trust.
Beyond Regulation: The Perils of Unbridled Uploads in Digital Pathology
The allure of bypassing quality control (QC) checks and directly uploading digital pathology slides to an image management system (IMS) might seem enticing in the face of increasing digital adoption. However, this seemingly convenient shortcut harbors a multitude of perils that extend far beyond mere regulatory non-compliance. Unvetted slides unleash a cascade of operational and clinical
risks, jeopardizing patient care, laboratory finances, and the integrity of the entire digital pathology ecosystem.
Misdiagnoses and Delays: A Domino Effect of Errors
The most concerning consequence of bypassing QC is the potential for misdiagnoses and delayed diagnoses. Unidentified artifacts or staining inconsistencies can masquerade as pathological features, leading pathologists down an erroneous diagnostic path. This can have devastating downstream effects, from missed cancers and misclassified tumor grades to inappropriate treatment decisions. The ripple effects of such errors are profound, causing unnecessary anxiety, delaying critical interventions, and potentially impacting long-term patient outcomes. Furthermore, the time wasted deciphering artifacts and wrestling with image inconsistencies translates to precious minutes diverted from actual diagnoses, adding unnecessary stress to an already vulnerable population.
A False Economy: Cost Avalanche in Disguise
Skipping local QC on digital pathology slides might seem like a time-saving measure, but it quickly transforms into a cost avalanche. Storing unusable images in the cloud consumes valuable storage space, incurring recurring fees for data that holds no diagnostic value. This is just the tip of the iceberg. Pathologists grappling with blurry artifacts or missing tissue samples expend precious minutes deciphering inconsistencies, adjusting settings, and navigating stitching errors – time that could be dedicated to actual diagnoses. This inefficiency cascades through the workflow, potentially delaying patient care and impacting treatment
timelines. The potential for misdiagnoses due to undetected issues further compounds the financial burden, potentially necessitating additional tests and procedures. Finally, consider the regulatory risk. Non-compliance with data integrity regulations can lead to hefty fines and reputational damage, adding another layer to the risk and financial burden.
Data Integrity: The Ethical Imperative
Beyond the immediate operational and financial concerns lies a more fundamental issue: data integrity. Uploading flawed data to the cloud contaminates the entire system, posing a significant risk to both clinical decision-making and research endeavors. Compromised data can lead to inaccurate diagnoses and misinterpretations, potentially impacting patient care and ultimately undermining the credibility of research findings. Prioritizing data quality through robust QC measures is not merely a financial consideration; it is an ethical imperative in safeguarding patient well-being and ensuring the validity of scientific knowledge.
In conclusion, the allure of bypassing QC in digital pathology is a dangerous illusion. The hidden costs, both financial and human, far outweigh any perceived convenience. Investing in a robust local QC process is not a burden, but an investment in a streamlined, efficient, and cost-effective digital pathology workflow, where patient safety and data integrity reign supreme.
Case Study: Blurred Lines and Missed Malignancy – Auto-Upload in Digital Pathology
Setting: A bustling urban hospital recently embraced digital pathology, opting for automatic upload of scanned slides to the cloud for analysis by a remote team of pathologists.
The Blurred Picture: A 45-year-old woman with a family history of breast cancer underwent a routine mammogram revealing suspicious calcifications. A core needle biopsy was performed, digitized, and auto-uploaded to the cloud. Unfortunately, a rare stitching error during the scanning process resulted in an area which was blurry, obscuring crucial details in the tissue architecture.
Remote Misdiagnosis: The remote pathologist, unaware of the image degradation, relied on the compromised digital file for diagnosis. Based on the hazy presentation, the pathologist skipped the area and concluded the presence of benign atypical hyperplasia.
Delayed Diagnosis and Devastating Outcome: The patient, reassured by the benign diagnosis, delayed further intervention. Months later, during a follow-up mammogram, the calcifications revealed concerning growth. A repeat biopsy confirmed the presence of invasive breast cancer, now at a more advanced stage.
Unraveling the Error: Upon investigation, the stitching error and its impact on image quality were identified. Had the local lab performed a basic manual QC check before upload, the blurry image would have been flagged, prompting rescanning and potentially a more accurate initial diagnosis.
Consequences of Auto-Upload Oversight: This case exemplifies the perils of prioritizing convenience over thoroughness in digital pathology. The reliance on auto-upload without local QC checks resulted in:
• Delayed diagnosis: The blurry image obscured the true extent of the malignancy, leading to a months-long delay in definitive diagnosis and treatment initiation.
• Missed opportunity for early intervention: Early detection and treatment of breast cancer are crucial for optimal prognosis. The auto-upload oversight potentially compromised the patient’s chances for a more favorable outcome.
• Erosion of trust: The misdiagnosis and delayed intervention could erode patient trust in both the medical system and the new digital pathology technology.
Lessons Learned: Local QC remains crucial. Regardless of the adopted workflow, local QC checks are essential for ensuring image quality and mitigating the risk of misinterpretations due to technical issues or artifacts.
Invest in training and infrastructure: Pathologists and lab personnel require training in identifying and managing image quality issues in the digital realm. Additionally, investing in reliable technology and robust scanning protocols can minimize technical errors.
A Blueprint for Success: Implementing Robust QC Measures:
To mitigate these risks and navigate the regulatory landscape with confidence, laboratories providing WSI must prioritize a meticulous QC process, even if it necessitates a manual approach before embracing automation. This process should encompass:
- Dedicated local storage: Allocate a separate local server or NAS for initial image storage, separate from the cloud IMS, to facilitate QC without compromising cloud storage capacity.
- Trained personnel: Assign QC tasks to designated, adequately trained personnel, such as pathologists or technicians, equipped with clear expectations and established standards.
- Visual inspection: Implement a rigorous visual inspection protocol for each image, evaluating focus, clarity, tissue coverage, artifacts, labeling accuracy, and image integrity.
- Standardized documentation: Maintain detailed records of QC findings and decisions in a standardized format to ensure auditability and transparency.
- Clear QC decisions: Categorize images as QC-approved for cloud upload, require rescans for correctable issues, or reject unusable images for clinical purposes.
- Secure transfer: Implement robust security measures to protect patient confidentiality during local storage and cloud transfer.
From Manual to Automation: Embracing the Future of QC:
While a manual QC process provides a solid foundation, it is crucial to acknowledge its limitations, particularly for larger workloads. Exploring and implementing automated QC software as it matures will streamline the process, enhance efficiency, and reduce human error. Additionally, tiered storage options within the cloud IMS can optimize resource allocation, minimizing storage costs associated with approved slides.
Conclusion:
Prioritizing a robust QC process before cloud upload is not simply a best practice; it is a non-negotiable imperative for navigating regulations and avoiding rookie mistakes in the digital pathology landscape. By investing in meticulous QC measures, laboratories can unlock the true potential of this transformative technology while ensuring patient safety, operational efficiency, and regulatory compliance. Quality control is an ethical imperative for safeguarding patient well-being and ensuring the accuracy and timeliness of diagnoses in the digital pathology landscape. Embracing a culture of quality-first ensures that we avoid the pitfalls of unbridled cloud uploads and emerge as true digital pathology professionals, building a future characterized by reliability, efficiency, and ultimately, patient-centered care.
Scott Kilcoyne, DigitCells